
24 September 2020
Travel out of Australia
Travel out of Australia continues to be a challenge with the Department of Home Affairs and Australian Boarder Force still restricting international travel.
Applications for Travel restriction exemption for travellers to and from Australia can be made here.
MTAA recommends applications include:
- A detailed itinerary with clear plans for quarantine (where applicable);
- This should include a guarantee that the applicants will adhere to relevant procedures and the company will cover the cost of their quarantine upon return
- A letter from the customer (in most cases the hospital CEO);
- This should include why the individual(s) and the work is essential.
- A letter from their employer outlining their role.
- This should include why the work can not be done by a person already at the destination
Please contact MTAA if you encounter any difficulties with these requirements.
Travel into New Zealand
Requests for travel into New Zealand can present some challenges particularly regarding the bulk of information required.
MTAA is working in collaboration with MTANZ to make this process more achievable.
If members can provide MTAA with the below information this can be provided to MTANZ who have direct contacts within New Zealand departments.
- Company Name:
- Technician(s) Name, DOB and Nationality:
- Technician(s) job title and brief description of area of expertise:
- Specific task/assignment (e.g. repair, installation, maintenance):
- Supporting documentation from the DHB or public health sector organisation (e.g. email, letter, works or purchase order):
- Any previously submitted documentation/correspondence relating to this specific application:
Where information is commercially sensitive it is requested the above contains as much detail as possible with sensitive lines (such as cost) redacted.
Interstate Travel Update
SA:
Travel between NSW and South Australia reopened to normal as of 24 September 2020. South Australia’s restrictions with Victoria remain unchanged.
QLD:
New border restrictions are now in place, as of 1am, 5 September 2020 details of the directions are available here.
Under Schedule 1, Section 2 part 3 of the Border Restriction Direction No.13 persons who, in carrying out their duties, are responsible for providing critical health support services for the critical maintenance, resupply or repair of health services infrastructure critical to Queensland are required to have:
evidence of Government or employer-issued identification or an official letter from an employer confirming employment;
- a letter confirming that the employee is required in Queensland. This is to be from the facility where the work will be undertaken or an appropriate officer of QLD Health. (from the hospital if private)
- It must be on a letterhead, and must include detail as to why it is essential and that nobody in Queensland can perform the service.
Persons under Section 1.2.3 are not required to complete 14 days mandatory quarantine in a government nominated hotel but are required to comply with the requirements in Schedule 1 of the direction, namely:
A person entering Queensland for an essential activity must only remain in Queensland for the time necessary to carry out the activity and must:
- keep and retain written records of close contacts for a 14-day period commencing on their date of arrival in Queensland, or, if they remain in Queensland for a period of less than 14 days, for that period; and
- provide the records to an emergency officer (public health) if directed by an emergency officer (public health) or to a contact tracing officer; and
- minimise contact with the community for a period of 14 days; and
- to the extent reasonably practicable, practice physical distancing including by remaining at least 1.5 metres from other people.
Applications for a Queensland Border Declaration Pass (Essential Activity) can be made here.
VIC:
Restrictions that began at 11:59pm Wednesday 5 August have been extended.
Employers that require their staff to attend a work site must issue a Permitted Worker permit to their employees – this is the employer’s responsibility.
For more information on eligibility and to apply for a permit, please click here.
MedTech employees who are required to access healthcare facilities are eligible to do so under the ‘all sectors’ category of Stage 4 Restrictions:
In addition to the Permitted Worker permit, we continue to recommend that all MedTech employees carry with them at all times:
- A letter from the customer (in most cases the hospital CEO); and
- A letter from their employer outlining their role.
We also recommend that you seek clarification from the customer regarding specific PPE requirements.
A dedicated Industry Coordination Centre has been set up within the Department of Jobs, Precincts and Regions to support businesses and consider ‘grey area’ cases to determine if businesses can safely operate under Stage 4 restrictions.
If you have further questions about the Permitted Workers Scheme please call Business Victoria on 13 22 15.
Please contact MTAA if you encounter any difficulties with these requirements.
International Freight Assistance Mechanism
Austrade are happy to confirm new and extended flight capacity from Melbourne to Narita and Melbourne to Los Angeles return.
The table below provides a summary of all new and extended flights announced by the International Freight Assistance Mechanism (IFAM) in the past fortnight.
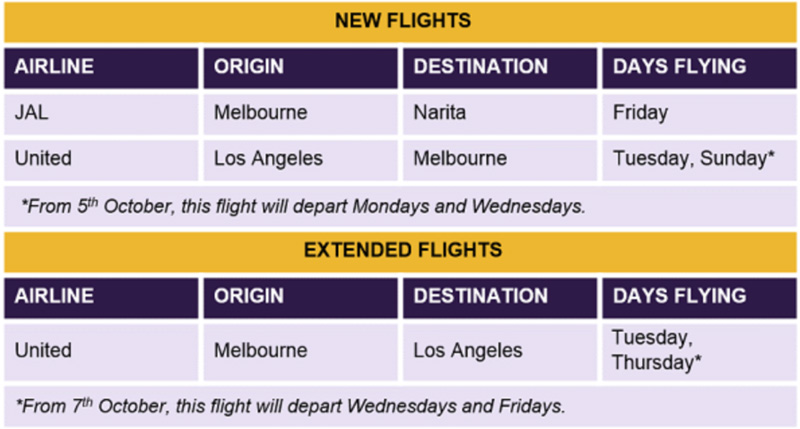
Full details can be found in the IFAM Flight Schedule Outbound and IFAM Flight Schedule Inbound.
IFAM Virtual Briefings
IFAM regularly holds state-based virtual industry briefings to provide updates. If you would like to join the invitation list, please email freightbriefing@austrade.gov.au with:
- your name
- business name
- State
Hand Sanitiser Industry Benchmark (for non-therapeutic goods)
Accord, the industry association representing manufacturers and suppliers of hygiene, personal care and specialty products, has produced a document outlining key information for manufacturers, suppliers and users of hand sanitisers. The Hand Sanitiser Industry Benchmark sets out the essential requirements to meet consumer expectations on efficacy, quality and safety of non-therapeutic, antibacterial hand sanitisers supplied in Australia, regardless of where the products are manufactured. It is intended to be read by a wide audience, and while jargon is avoided wherever possible, the document does contain references to regulations and technical standards.
This Hand Sanitiser Industry Benchmark outlines the:
- Purpose of the document,
- Scope, or the products to which this document applies,
- minimum requirements that ingredients in hand sanitisers must meet for safety and efficacy,
- minimum requirements that the final product must meet for safety and efficacy,
- minimum labelling requirement, over and above the existing regulatory requirements, and
- acceptable claims and the evidence required to support the claims.
Further information can be found on Accord’s website here.
A copy of the Hand Sanitiser Industry Benchmark can be found here.
28 August 2020
Department of Industry Supply Chain Roundtable Survey
The AusIndustry network, of which MTAA is a member and Treasury’s Business Liaison Unit have identified the following ongoing challenges:
- Border closures are impacting business continuity.
- Actions of governments in response to the COVID threat are difficult to predict and affecting the outlook of business.
- Businesses have difficulty navigating guidance/rules from governments and regulatory processes.
- Compliance with COVID-safe health requirements comes with significant cost.
- Stress and mental health impacts for business leaders and workers struggling to manage/work.
- International markets are inaccessible due to constrained and costly freight services.
As part of the roundtable process the AusIndustry network and Treasury’s Business Liaison Unit are asking the below questions. MTAA will need to feed any responses back to the working group by Wednesday 2 September 2020 so if responses could be provided to MTAA via email to estrong@mtaa.org.au prior to the 2nd September, it would be greatly appreciated.
Your Feedback Needed
Question | Response |
· Are there additional challenges to those listed that will play out over the medium term (18 months - 2 years). Are these particular to your industry sector? |
|
· What are the top 5 challenges you consider will be a significant issue over the medium term? |
|
· Can you offer 3-5 pertinent examples of how these issues are playing out on the ground? |
|
· What are your suggested mitigations or solutions to address these challenges? |
|
· What can your organisation do to support members and industry? |
|
· Is your membership changing how it does business to mitigate the COVID threat and associated impacts? |
|
· Do you have any other input? |
|
Interstate Travel Update
VIC:
Restrictions that began at 11:59pm Wednesday 5 August remain in place in Victoria.
From 11:59pm on Wednesday 5 August, employers that require their staff to attend a worksite must issue a Permitted Worker permit to their employees – this is the employer’s responsibility.
For more information on eligibility and to apply for a permit, please click here.
MedTech employees who are required to access healthcare facilities are eligible to do so under the ‘all sectors’ of Stage 4 Restrictions:
In addition to the Permitted Worker permit, we continue to recommend that all MedTech employees carry with them at all times:
- A letter from the customer (in most cases the hospital CEO); and
- A letter from their employer outlining their role.
We also recommend that you seek clarification from the customer regarding specific PPE requirements.
A dedicated Industry Coordination Centre has been set up within the Department of Jobs, Precincts and Regions to support businesses and consider ‘grey area’ cases to determine if businesses can safely operate under Stage 4 restrictions.
If you have further questions about the Permitted Workers Scheme please call Business Victoria on 13 22 15.
Please contact Edward Strong on 0437 629 884 or estrong@mtaa.org.au if you encounter any difficulties with these requirements.
IFAM airfreight service provider update
Air New Zealand and United Airlines have joined the International Freight Assistance Mechanism (IFAM) airfreight service provider panel. Both airlines will be supporting new flights to the USA.
For further details on the airfreight service provider panel and new USA flights, please see the IFAM webpage.
IFAM have also confirmed the following new and extended flight capacity on the following routes:
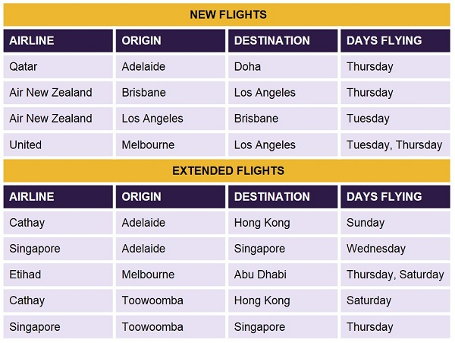
Full details can be found in the IFAM Flight Schedule Outbound and IFAM Flight Schedule Inbound.
Exporters still need to submit an Expression of Interest (EOI) and should keep in regular contact with freight forwarders to access IFAM-supported flights. Further information on how IFAM works is available on the. IFAM webpage.
20 August 2020
General recommendations
MTAA recommends that MedTech employees carry with them at all times, as a minimum:
- Physical copies of any documentation specifically required by the state to which they are traveling;
- A letter from their employer; and
- A letter from the relevant customer (ie the hospital to which they are travelling).
MTAA is able to provide a letter confirming MTAA membership and completion of OTP course if required. We can also provide a template letter to assist with identification purposes for staff of member companies. Please contact Peter Tustin for further assistance: ptustin@mtaa.org.au.
NSW
New border entry rules from August 7 – NSW/VIC: https://www.nsw.gov.au/covid-19/what-you-can-and-cant-do-under-rules/border-restrictions
A transit permit is required to enter NSW if you've been in Victoria in the last 14 days, except if you're entering NSW:
- as an emergency or law enforcement services worker
- to access emergency medical, hospital, dental or veterinary care
- to avoid injury or escape a risk of harm.
The permit is valid for 3 days from the date of issue.
In almost all cases, permits are issued subject to conditions. The permit will indicate if you need to:
- go into mandatory hotel quarantine for 14 days
- self-isolate for 14 days
- get tested for COVID-19
- abide by a COVID-19 safety plan or
- comply with any other conditions.
NSW border entry permit: https://www.service.nsw.gov.au/transaction/apply-covid-19-nsw-border-entry-permit
Registration for critical services worker for a COVID-19 NSW border entry permit: https://www.service.nsw.gov.au/transaction/register-critical-services-worker-covid-19-nsw-border-entry-permit
QLD
New border entry rules from August 8 - entry to Queensland is currently only permitted in accordance with the Border Restrictions Direction (No 11), which may require additional documentation, and a completed Queensland Border Declaration Pass.
To cross the border you will need to obtain a Queensland Border Declaration Pass. Applications can be made at the border, however you may face delays
All of Victoria, New South Wales and the Australian Capital Territory are now COVID-19 hotspots. COVID-19 hotspots are updated regularly here.
Returning travellers will need to quarantine for 14 days: https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19/protect-yourself-others/quarantine
Exemptions are available, but not many are being granted currently: https://www.health.gov.au/contacts/queensland-government-covid-19-quarantine-exemptions-contact.
NSW/QLD border bubble
Waiver for mandatory 14 day quarantine requirement can be applied for at https://qldsurgeryconnect.service-now.com/hdsp
Current exemptions can be found at https://www.health.qld.gov.au/system-governance/legislation/cho-public-health-directions-under-expanded-public-health-act-powers/border-restrictions
The current postcodes that are included within the QLD/NSW ‘bubble’ can be found at https://www.health.qld.gov.au/__data/assets/pdf_file/0026/998000/map-border-zones-qld-border-restriction-direction.pdf
NSW/VIC border zone
Information for critical service workers in the border region: https://www.nsw.gov.au/covid-19/what-you-can-and-cant-do-under-rules/border-restrictions/areas#critical-service-workers-in-the-border-region
Border zone address check: https://www.service.nsw.gov.au/border-zone-address-check
VIC
From 11:59pm Wednesday 5 August, workplaces in Melbourne must be closed unless:
- the workplace is part of a permitted activity, or
- all employees are working from home.
From 11:59pm on Wednesday 5 August, employers that require their staff to attend a work site must issue a Permitted Worker permit to their employees – this is the employer’s responsibility.
For more information on eligibility and to apply for a permit, please click here.
MedTech employees that are required to access healthcare facilities are eligible to do so under the ‘Health care and social assistance’ category of Stage 4 Restrictions.
SA
From 12:01 am Wednesday 29 July, only essential travellers will be able to enter SA from Victoria. South Australians will no longer be able to return to SA from Victoria.
All visitors are still required to register online up to 72 hours earlier.
Travellers still need to self-quarantine whilst in the state and wear a mask in public.
To qualify as Essential Skills (industries and businesses), you will need a letter detailing that the person is required to conduct a service that is time-critical and where the provision of the service requires that the person be physically present in South Australia.
Travellers will need to qualify under one of the following categories:
- Specialists required for industry or business continuity and maintenance of competitive operations where the appropriate skills are not available in South Australia, where the service is time-critical and where the provision of the service requires that the person be physically present in South Australia.
- Persons who, in the conduct of their duties, are responsible for maintenance or repair of infrastructure critical to South Australia and are required to be physically present in South Australia for such purposes.
You must provide a letter from your employer or other documentation in support of your application.
On arrival in South Australia:
- You are required to self-quarantine at all times when you are not undertaking, duties, functions or activities associated with your essential traveller status or travelling to or from such duties, functions or activities.
- You are required to wear a surgical face mask (covering mouth and nose) when coming in to contact with members of the public.
Further information is available here.
WA
As of August 17 you cannot enter WA unless you are an exempt traveller.
Exemptions are set out under the Quarantine (Closing the Border) Directions and can be applied for under the G2G PASS. Claims are currently being processed within 6 days.
Alternatively, you can submit an exemption application form via email with supporting documentation. These paper-based application forms take longer to process. If you choose to submit an application using the WA Entry Form, please submit your application at least 4 weeks prior to travel.
You should receive an email from noreply@mail.g2gpass.com.au once your application has been received.
You should ensure you receive approval to travel from WA Police before entering WA.
You will need to carry evidence of this approval with you, when you travel. If you have a G2G Pass, your unique QR code can be scanned at border checkpoints for this purpose. Authorised officers will scan the code to confirm you are travelling for your approved purpose.
If your exemption category requires further documentation or proof, you must produce this on request. Failure to do so may result in your application being refused.
TAS
Please be advised that due to NSW updating their border restrictions for travel from Victoria, entry to the ACT from Victoria is now only possible through Canberra Airport, by air.
Under a Public Health Direction, anyone (other than ACT residents) travelling into the ACT from Victoria will be denied entry to the ACT unless they are granted an exemption by ACT Health.
Applications should be submitted as early as possible, and at least 72 hours before the intended travel date, wherever possible.
If it has been more than 14 days since you have been in Victoria, you will not require an exemption to enter the ACT.
Additional contact details: COVID.exemptions@act.gov.au
ACT
Please be advised that due to NSW updating their border restrictions for travel from Victoria, entry to the ACT from Victoria is now only possible through Canberra Airport, by air.
Under a Public Health Direction, anyone (other than ACT residents) travelling into the ACT from Victoria will be denied entry to the ACT unless they are granted an exemption by ACT Health.
Applications should be submitted as early as possible, and at least 72 hours before the intended travel date, wherever possible.
If it has been more than 14 days since you have been in Victoria, you will not require an exemption to enter the ACT.
Additional contact details: COVID.exemptions@act.gov.au
NT
There are strict border controls in place for all arrivals to the Territory, including returning Territorians.
All arrivals to the Northern Territory (NT) must:
- fill in a Border Entry Form
- complete 14 days of mandatory supervised quarantine at your own expense*, if you have recently been in an active declared COVID-19 hot spot.
Information on exemptions available here.
For further information regarding exemptions, you can call 1800 490 484.
5 August 2020
VIC update
From 11:59pm Wednesday 5 August, workplaces in Melbourne must be closed unless:
· the workplace is part of a permitted activity, or
· all employees are working from home.
From 11:59pm on Wednesday 5 August, employers that require their staff to attend a work site must issue a Permitted Worker permit to their employees – this is the employer’s responsibility.
For more information on eligibility and to apply for a permit, please click here.
MedTech employees that are required to access healthcare facilities are eligible to do so under the ‘Health care and social assistance’ category of Stage 4 Restrictions:
Any ancillary business (including IT) involved in the production, supply, manufacture, repair, maintenance, cleaning, security, wholesale, distribution, transportation or sale of equipment, goods or services necessary for the operations of a permitted work site or for closed work sites where there are safety or environmental obligations.
In addition to the Permitted Worker permit, we continue to recommend that all MedTech employees carry with them at all times:
· A letter from the customer (in most cases the hospital CEO); and
· A letter from their employer outlining their role.
We also recommend that you seek clarification from the customer regarding specific PPE requirements.
Please contact Rachel Fry on 0417 887 432 or rfry@mtaa.org.au if you encounter any difficulties with these requirements.
24 July 2020
NSW - new mask requirements in healthcare facilities
Given the current context of local transmission, NSW Health has advised LHDs / SCHNs to escalate to a moderate risk level (Amber). This requires all health workers wear a surgical mask if they are within 1.5m of patients. Patients are also required to wear a mask, where possible. This advice applies to hospital and community health settings and comes into effect from Friday 24 July 2020.
Read more here.
We will provide additional information on this as it comes to hand.
SA Travel update
From 12:01 am Wednesday 29 July, only essential travellers will be able to enter SA from Victoria. South Australians will no longer be able to return to SA from Victoria.
All visitors are still required to register online up to 72 hours earlier.
Travellers still need to self-quarantine whilst in the state and wear a mask in public.
To qualify as Essential Skills (industries and businesses), you will need a letter detailing that the person is required to conduct a service that is time critical and where the provision of the service requires that the person be physically present in South Australia.
Travellers will need to qualify under one of the following categories:
- Specialists required for industry or business continuity and maintenance of competitive operations where the appropriate skills are not available in South Australia, where the service is time critical and where the provision of the service requires that the person be physically present in South Australia.
- Persons who, in the conduct of their duties, are responsible for maintenance or repair of infrastructure critical to South Australia and are required to be physically present in South Australia for such purposes.
You must provide a letter from your employer or other documentation in support of your application.
On arrival in South Australia:
- You are required to self quarantine at all times when you are not undertaking, duties, functions or activities associated with your essential traveller status or travelling to or from such duties, functions or activities.
- You are required to wear a surgical face mask (covering mouth and nose) when coming in to contact with members of the public.
Further information is available here.
New and extended IFAM supported flights
As part of phase 2, the International Freight Assistance Mechanism (IFAM) has announced the following new and extended flights. Also, all flights are now open 72 hours prior to departure for all freight forwarders to access.
New IFAM Outbound supported flights:
- Sydney to Shanghai with Qantas
Extended IFAM Outbound supported flights:
- Adelaide to Singapore with Singapore Airlines
- Brisbane to Singapore with Qantas
- Brisbane to Narita with Qantas
- Brisbane to Auckland with Qantas
- Brisbane to Hong Kong via Cairns with Qantas
- Brisbane to Los Angeles with Virgin
- Darwin to Brisbane with Toll
- Melbourne to Hong Kong with Qantas
- Melbourne to Singapore with Qantas
- Melbourne to Narita with Japan Airlines
- Sydney to Narita with Qantas
- Sydney to Narita with Japan Airlines
- Toowoomba to Hong Kong with Cathay Pacific
- Toowoomba to Singapore with Singapore Airlines
Extended IFAM Inbound supported flights:
- Auckland to Brisbane with Qantas
- Hong Kong to Brisbane with Qantas
- Hong Kong to Melbourne with Qantas
- Los Angeles to Brisbane with Virgin
- Narita to Sydney with Qantas
- Narita to Brisbane with Qantas
- Narita to Melbourne with Japan Airlines
- Singapore to Melbourne with Qantas
- Singapore to Brisbane with Qantas
Full details of all available IFAM-supported flights can be found in the IFAM Flight Schedule Outbound and IFAM Flight Schedule Inbound.
8 July 2020
Travel between NSW and VIC
As of midnight last night, the NSW/VIC border has been closed. The NSW Government has issued health advice for people who have travelled to NSW from Victoria or Melbourne in the last 14 days and managing those in high-risk settings.
On its website, Services NSW has provided information and established a process for applying for a permit to travel from Victoria into NSW for exempted categories.
People covered by the permit include a person providing critical services, including:
- freight and logistics
- maintenance and repair of critical infrastructure
- medical or hospital care
The website currently notes that high volumes of applications mean there are delays in processing permit applications and an alternative is to carry sufficient paperwork to demonstrate you are in an exemption category as above.
Advice for staff of health facilities who have returned from greater Melbourne
All persons entering a health facility will be:
- assessed for symptoms and fever and asked to be tested and self-isolate if symptomatic
- asked if they have travelled from greater Melbourne metropolitan area in the last 14 days
If they have travelled from greater Melbourne metropolitan area in the last 14 days then:
- the will be advised that should even the mildest of symptoms arise, they must self-isolate and be tested for COVID-19
- the will be asked if they have been in a hotspot. If they have been in a hotspot then they will be asked to self-isolate and be excluded from work, except in exceptional circumstances
- exceptional circumstances include where the absence of the staff member will significantly compromise clinical care. In this case, assess the risk of COVID infection against the consequence of impacting patient care, and whether the risk can be adequately mitigated, with consideration given to:
- the location and duration of contact in COVID-19 community outbreaks areas in Melbourne, and nature of the transmission risk involved (e.g. number of social contacts, level of contact with potentially affected communities);
- the type of clinical duties undertaken, and whether other mitigation strategies such as physical distancing, hand hygiene and use of PPE will be possible at all times.
The full fact sheet is available here.
We will provide additional updates as they become available, in the meantime, please contact rfry@mtaa.org.au if you have any queries regarding this.
Elective Surgery – VIC
Victorian Health Minister Jenny Mikakos advised yesterday that elective surgery will be allowed to continue in Victoria.
Freight update: IFAM extended
On 3 July, the Australian Government committed an additional $241.9 million to continue the International Freight Assistance Mechanism (IFAM), helping keep international freight routes and flights operating until the end of the year. The new funding will keep high-value, time-sensitive and perishable exports and vital imports flowing as we continue the economic recovery from the impact of COVID-19 on global supply chains. It will also re-establish domestic connections for producers and growers in regional and rural areas that rely on air freight to get their products to customers.
More information on the program’s extension will be provided at a Virtual Briefing Session:
IFAM Briefing:
Thursday, 9 July
11.30 am – 12.15 pm (AEST)
(Please dial in from 11.25 am for a prompt start at 11.30 am)
Please dial 02 9037 0069 and enter the meeting number (access code) if prompted: 166 786 1721#
OR
If opening on a mobile, tap this number: +61-29037-0069 and enter 1667861721#
You will be automatically muted on entry to this call.
There will be an opportunity to ask questions during the meeting. Please press *6 to unmute.
1 July 2020
Essential traveller update – South Australia
From July 1 visitors to SA will need to apply for pre-approval 72 hours prior to entering the state. They will then be told if they need to quarantine or not. The SA Premier has advised that the state will not open its border with Victoria on July 20. For more information, please go to: https://www.police.sa.gov.au/online-services/cross-border-travel-application.
26 June 2020
Essential traveller update - SA
Arriving from interstate:
From NT, QLD, TAS and WA
The SA government has eased border restrictions for people entering South Australia from:
- Northern Territory
- Queensland
- Tasmania
- Western Australia.
Travellers entering South Australia directly from these states (including NT) are no longer required to quarantine for 14 days. Anyone who arrived directly from these states and was ordered to quarantine, is no longer required to quarantine, regardless of how long they have been in SA.
This only applies to travellers arriving directly from these states and territory. Travellers arriving from all other states and territories are still required to quarantine for 14 days. For example, if you are “from” WA, Tasmania or NT but arrive from Victoria or NSW, you will still need to quarantine for 14 days.
Changes to essential traveller rules:
Staff providing technical services are now categorised as an essential traveller category 2 which requires travellers to quarantine for the period of 14 days, and only leave to undertake their essential work. This is a change from previous arrangements where there was only one category and all essential travellers were exempt.
If coming from Queensland (from 20 June), the Northern Territory, Tasmania or Western Australia – no quarantine required.
However if you are coming from these states/territory via NSW or Victoria you will be required to quarantine.
More information, including changes to essential traveller rules, available here.
Freight update: New IFAM flights
New flights under the International Freight Assistance Mechanism (IFAM) are now available from Los Angeles to Melbourne with Virgin Australia.
There will be a new flight from Los Angeles to Melbourne every Wednesday, commencing 01 July, for six weeks. The Los Angeles to Melbourne flight departing on Saturdays has also been extended for further six weeks, commencing 04 July.
There is capacity inbound (to Australia) on these flights and medical and health supplies will be prioritised.
Pricing
Virgin is applying the following rates for the freight. These rates may be subject to change and will be reviewed weekly.
| Route | AUD / kg |
| LAX - MEL | $5.70 |
Please note: The above rates are for the air freight component only, they do not include freight forwarder fees or rates.
Other Available IFAM Supported Flights:
There is capacity on the following inbound IFAM routes:
- Tokyo (Narita) to Melbourne
- Tokyo (Narita) to Brisbane
- Tokyo (Narita) to Sydney
- Singapore to Brisbane
- Singapore to Melbourne
- Auckland to Brisbane
- Auckland to Melbourne
- Hong Kong to Brisbane
- Hong Kong to Melbourne
- Los Angeles to Brisbane
- Los Angeles to Melbourne
Please see the IFAM inbound flight schedule for all the details, and the Austrade webpage for the latest IFAM information.
19 June 2020
Current scheduled IFAM flights
The IFAM is currently supporting the following flights:
- Sydney to Tokyo (Haneda) one way
- Sydney to Tokyo (Narita) return
- Sydney to Dubai one way – NEW
- Brisbane to Los Angeles return
- Brisbane to Singapore return
- Brisbane to Tokyo (Narita) return
- Brisbane to Auckland return
- Brisbane to Cairns to Hong Kong return
- Brisbane to Dubai one way – NEW
- Melbourne to Tokyo (Narita) return
- Melbourne to Singapore return
- Melbourne to Hong Kong return
- Melbourne to Abu Dhabi one way
- Melbourne to Auckland return
- Adelaide to Singapore one way
- Adelaide to Hong Kong one way
- Toowoomba to Hong Kong one way
- Toowoomba to Singapore one way
Domestic links to connect with international IFAM freight flights:
- Hobart to Sydney one way
- Darwin to Brisbane one way
Visit the IFAM webpage for the latest flight information and schedules.
17 June 2020
12 June 2020
MTAA secures TGA fee relief for members
Following a sustained advocacy effort by MTAA, the Federal Government has agreed to significant fee relief for medical device companies, in recognition of the impact of elective surgery cancellations as a result of the impact of COVID-19.
A 50 per cent reduction in annual TGA listing fees for Class IIa, IIb, III or AIMD medical devices listed on the Prostheses List will apply for 2020-21.
This will reduce the annual charges for those devices for 2020-21 by 50 per cent of the amount that would have been otherwise applied to them under the proposed 1.95% increase for 2020-21.
We would like to thank those member companies who have contributed their time and resources to assisting MTAA in delivering this important outcome for members.
Return to elective surgery webinar
The COVID-19 pandemic and the necessary measures taken to contain it have caused significant disruptions to our healthcare system. The cancellation of elective surgery in late March this year will have ongoing ramifications for the entire sector.
This webinar will bring together industry leaders from across private healthcare to discuss the current landscape and how to address the ongoing challenges presented by the return to elective surgery, including:
- How far has elective surgery returned to normal rates of effort?
- Constraints on returning to full capacity
- Key points from the National Health Agreement
- Risk management, including PPE Supply and staff safety
- Industry reps in theatre and how best to partner effectively with hospitals during this time to support their effort
- Lasting effects of COVID-19 for surgery
Date: 18 June 2020
Time: 14:00 - 15:00 AEST
Cost: Free livestream for member | $60 +GST for non-members
Freight update: New IFAM flights
The International Freight Assistance Mechanism (IFAM) is supporting new return flights to Los Angeles and Tokyo (Narita) and one-way flights to Singapore and Tokyo (Haneda). In addition, IFAM has extended key routes with Qantas to Shanghai, Tokyo, Singapore, Hong Kong and Auckland.
The IFAM is currently supporting the following flights:
- Brisbane to Los Angeles return - NEW
- Melbourne to Tokyo (Narita) return - NEW
- Sydney to Tokyo (Haneda) one way - NEW
- Toowoomba to Singapore one way - NEW
- Sydney to Shanghai one way - Extended
- Sydney to Tokyo (Narita) return - Extended
- Brisbane to Singapore return - Extended
- Brisbane to Cairns to Hong Kong return - Extended
- Brisbane to Auckland return - Extended
- Melbourne to Singapore return - Extended
- Melbourne to Hong Kong return - Extended
- Melbourne to Auckland return - Extended
- Adelaide to Hong Kong one way
- Toowoomba to Hong Kong one way
- Melbourne to Abu Dhabi one way
- Adelaide to Singapore one way
Domestic links to connect with international IFAM freight flights:
- Hobart to Sydney one way
- Darwin to Brisbane one way - NEW
Please visit the IFAM webpage for the latest flight information and schedules.
Export Finance Australia - COVID-19 support
If your business has been affected by COVID-19 and you need finance, Export Finance Australia is there to help.
They provide a range of finance solutions for exporters or businesses in an export-related supply chain.
If you have encountered any shipment problems, payment difficulties or business interruptions and are unable to secure finance due to COVID-19, Export Finance Australia may be able to assist.
Finance solutions:
- Working capital support: to finance against supplier invoices or international purchase orders
- Capital investment: to purchase new equipment and expand your export operations
- International expansion: to establish or grow your business operations overseas
- Online growth: to invest in eCommerce and grow your sales to international customers.
29 May 2020
COVID-19 Manufacturer Response Register
The Government's Advanced Manufacturing Growth Centre (AMGC) has developed a COVID-19 Manufacturer Response Register to bring together manufacturers, suppliers and customers via a self-managed platform. Read more here. The register can be accessed here.
NSW Government – NSW Business COVID-19 Research
The NSW Small Business Commissioner is conducting a survey on the impact of COVID-19 for businesses across NSW. The Survey aims to provide the NSW Government with an understanding of the challenges businesses are facing and the level of awareness of assistance available to help them now and post-COVID-19. You can participate in the survey here.
Roadmap to a COVIDSafe Australia
The Australian Chamber of Commerce and Industry have published a new quick reference document for businesses operating across jurisdictions. This PDF maps all state and territory stepped plans across the high-level 3-step framework national Cabinet released. Download the PDF here.
Freight Update - Melbourne–Abu Dhabi flight now available
The IFAM is now supporting outbound flights between Melbourne and Abu Dhabi. The IFAM is currently supporting the following flights:
- Melbourne to Abu Dhabi one way - NEW
- Sydney to Shanghai one way
- Sydney to Tokyo (Narita) return
- Brisbane to Singapore return
- Brisbane to Cairns to Hong Kong return
- Brisbane to Auckland return
- Melbourne to Singapore return
- Melbourne to Hong Kong return
- Melbourne to Auckland return
- Adelaide to Singapore one way
Please visit the IFAM webpage for the latest flight information and schedules.
Continuity of Care Collaboration
MTAA has come together with over 20 of Australia’s leading healthcare organisations and associations to pen an open letter to Australians about the need to put their health first. Read the letter here.
New Austrade COVID webpage
Austrade have a new dedicated webpage - COVID-19: Support for Australian businesses, including links to recordings of previous webinars and a forward schedule of webinars.
Australian Government links to COVIDSafe and other business resources
Click here to access Australian Government resources for promoting a COVIDSafe environment in your workplace. Both pre-made and customisable materials are available.
You can access the Continuing your business page here.
14 May 2020
Freight update
Additional flights have been arranged through the International Freight Assistance Mechanism (IFAM).
There is capacity inbound from Auckland, Hong Kong, Tokyo (Narita) and Singapore in which medical supplies will be prioritised.
Please see the Qantas flight schedule and Singapore Airlines schedule for flight numbers, days of operation, departure and arrival times.
Members will need to work with their freight forwarders in order to access freight space. If members require additional assistance and suggestions of freight forwarders based with medical and market expertise, Austrade is able to provide this.
For any further assistance or questions, please feel free to contact or Austrade directly at airfreight@austrade.gov.au.
Essential Traveller Update – QLD
Due to changes in restrictions, returning QLD residents must apply online for a Queensland Entry Pass, which can be accessed here. The new passes are applicable to a person rather than a vehicle, as was the case with the previous pass.
Government releases Roadmap to a COVIDSafe Australia
The Federal Government has released ‘Roadmap to a COVIDSafe Australia’, a three-step pathway for easing restrictions. Read more here.
NCCC business COVIDSafe plan toolkit
The National Covid-19 Coordination Commission has released a guide for business to develop a COVIDSafe plan. The guide can be accessed here.
JobKeeper Payment Guide
ACCI has released an updated JobKeeper Payment Guide to assist employers. The information guide seeks to explain and answer some of the more common questions employers may have around the payment scheme and it can be accessed here.
Elective Surgery – South Australia to resume
South Australia will become the first state to begin to fully restore elective surgery with the lifting of the Appropriate Surgery Direction under the Emergency Management Declaration. Read media release here.
National Measurement Institute helping the health and manufacturing industry respond to COVID-19
The Department of Industry, Science, Energy and Resources has just published a news story on their website regarding the National Measurement Institute and how they are helping the health and manufacturing industries to respond to the COVID-19 pandemic.
You can view the full news story here.
Applications open for COVID-19 research grants
Guidelines and application details for round one of the NSW Heath COVID-19 Research Grants are now available on the NSW health and medical research website.
Round one applications close on 20 May 2020. Apply here.
Free and discounted training courses available to MTAA members
In response to the impact of COVID-19 on our members, we are providing a number of training courses free or at a 25% discount. Please see the MTAA website for more information.
6 May 2020
Essential Traveller update
MTAA is continuing to work on addressing issues faced by members around interstate travel restrictions, however, we have received feedback from a number of member companies that in most cases, restrictions can be addressed by providing the relevant documentation. We recommend that medtech employees carry with them, as a minimum:
- Physical copies of any documentation specifically required by the state to which they are travelling, links available on MTAA website;
- A letter from their employer; and
- A letter from the relevant customer (ie the hospital to which they are travelling).
MTAA is able to provide a letter confirming MTAA membership and completion of OTP course if required. We can also provide a template letter to assist with identification purposes for staff of member companies. Please contact Peter Tustin for further assistance: ptustin@mtaa.org.au.
Freight Update
On 23 April, the Government announced a new network of 15 air freight service providers and freight forwarders to accelerate the delivery of agricultural and fisheries exports to key overseas markets. On return, these flights will bring back medical supplies, medicines and equipment to support Australia’s COVID-19 response. The $110 million International Freight Assistance Mechanism (IFAM) is to provide assistance to exporters and re-establish global supply chains.
Further information on the International Freight Assistance Mechanism is available here. For more information about importing medical supplies, please contact airfreight@austrade.gov.au.
Austrade Medical Imports contact
Mrs Emily Bogue is the head of the office’s medical imports team and is working for the new International Airfreight Coordinator General. Her email contact is: Emily.Bogue@austrade.gov.au. There is also a general inbox: Airfreight@austrade.gov.au.
The Office of the International Airfreight Coordinator General is managing requests to assist in the import of medical goods and PPE including medical devices and their components for machines like ventilators and may be able to assist with international freight needs. The Office has specific templates to help match industry demand to available services that they are operating. The office also has embedded officers and logistics experts from Qantas and Virgin, and also operates a panel arrangement with a number of key freight forward operators and international airline contractors in the service of their program. If they are unable to assist with specific requests they may be able to connect people with appropriate industry contacts that can support commercial terms.
You can read the media release here.
MTAA freight working group
MTAA has established a specialist freight group as part of MTAA’s COVID-19 Working Group structure. The purpose of the group is to provide a summary of issues and freight route priorities to feed directly into Austrade’s weekly panel meetings.
Please contact Paul Dale if you would like to be part of this.
Update: ACCC interim authorisation
As part of MTAA’s COVID-19 effort, a conditional authorisation (AA1000479) was successfully obtained from the ACCC to enable industry to collaborate in supporting the Commonwealth to secure medical supplies throughout the COVID-19 pandemic.
The ACCC have advised MTAA that, as part of their review, they will be contacting the companies listed on the conditional authorisation (AA1000479) to request feedback on how the authorisation is working. MTAA encourages companies to provide a response, particularly those companies that are actively involved in the COVID-19 working groups.
NSW Government COVID-19 Emergency Supplies Portal
The NSW Government has recently launched the COVID-19 Emergency Supplies Portal. Businesses interested in sourcing hand sanitiser, examination gloves, disinfectant and cleaning products, handwash and soap, masks, eyewear, gowns and protective overalls and paper products including toilet and tissue paper, can access local PPE suppliers here. Businesses can continue to register their interest to supply here.
Standards Australia summary information on standards and conformance for PPE products
Standards Australia have recently released summary information on standards and conformance for PPE products in consultation with the Australian Technical Infrastructure Alliance, to assist in helping manufacturers identify relevant standards and find potential pathways to market. Read more here.
McKinsey paper on COVID & MedTech
McKinsey & Company have recently published a paper on COVID-19 and MedTech, Reimagining medtech for a COVID-19 world. You can read it here.
28 April 2020
Government assistance for the MedTech sector
MTAA wrote to Minister Hunt on 29 March outlining the very significant pressures facing the medical technology industry in the current environment and requesting a number of urgent measures to address the ongoing viability of Australia’s MedTech industry as the COVID-19 crisis continues.
The MTAA Board met with Health Minister Greg Hunt on Friday last week to discuss this and other matters, including a return to elective surgery and additional support measures for SMEs and Australian companies.
Following this meeting and a period of intense discussions between MTAA and Minister Hunt and his office, a number of measures have been confirmed by the Minister in relation to PL reform.
Jobkeeper update
MTAA has been liaising with the Australian Tax Office (ATO) in order to seek clarity on the Jobkeeper provisions available to medtech companies, particular in relation to small companies where they are a local branch of a global company.
The ATO have clarified that, in determining whether a company must show a 30% or a 50% reduction in turnover, the global revenue of the global entity of which it is a part is included. If the global entity exceeds $1b, at least a 50% reduction must be demonstrated to qualify.
However, once the threshold is set, the turnover test itself is based on local GST revenue, not global revenue. Therefore, a small company which is a local branch of a global company exceeding $1b in revenue in total would still need to show that Australian turnover has fallen more than 50% to qualify for JobKeeper.
If global revenues were less than $1b, the fall in Australian turnover would only need to be 30% to qualify.
Freight group – call for EOIs
MTAA is calling for expressions of interest from those interested in joining a specialist freight group as part of MTAA’s COVID-19 Working Group structure.
The purpose of the group would most likely be to provide a summary of issues and freight route priorities to feed directly into Austrade’s weekly panel meetings. It is envisaged that a representative from Austrade would be part of the group.
Please contact Paul Dale if you would like to be part of this.
21 April 2020
Elective Surgery to resume after Anzac Day
Prime Minister Scott Morrison has announced that elective surgery will begin to gradually re-start after the Anzac Day long weekend.
National Cabinet met this afternoon and agreed that category two surgeries and selected category three surgeries will start from Monday, with a review on May 11 to determine if and when more restrictions will be lifted.
Click here to read the Department of Health media release.
Essential Traveller update – South Australia
The South Australian Government has published an updated link to their essential traveller guidelines, available here. The guidelines now include a formal written clarification around ‘health services’.
2—Health services
Persons who are requested by the Chief Executive of the Department for Health and Wellbeing, or by the Secretary of the Commonwealth Department of Health, or by the delegate of either, to assist in the provision of health services in South Australia and who are required to be physically present in South Australia in the conduct of those duties.
Note—
This means that South Australian health care workers who wish to provide services in another State or jurisdiction will be required to undertake a 14-day quarantine period on their return to South Australia. The only exception is a person who falls within the terms of this clause.
If you require further information you can call the SA COVID-19 Information Line on 1800 253 787 between the hours of 8am to 8pm seven days a week or go to the SA Health website at www.sahealth.sa.gov.au or www.sa.gov.au/covid-19
EU-MDR Update
EU Parliament decides to postpone implementation of new Medical Devices Regulation by one year
On Friday April 17th the EU Parliament decided to postpone implementation of the new medical devices and in vitro medical devices regulations 2017/745 (MDR) and 2017/746 (IVDR) by one year. The new regulations entered into force in May 2017 with a transitional period for full enforcement of 3 years for the MDR (i.e. until May 2020) and 5 years for the IVDR (until May 2022).
Due to the current pressure on national health authorities, manufacturers and suppliers of medical devices to address COVID-19 priorities, the European Parliament has decided to postpone the deadline for full enforcement of the MDR by one year to allow authorities and manufacturers alike to prioritise the fight against the coronavirus pandemic. Read the full EU Parliament press release here.
The Australian policy is to remain aligned with the EU medical device regulations and TGA has already started to take steps aligning with the new EU MDR, such as adopting requirements for patient implant cards and information leaflets and up-classifying certain medical devices. As the majority of medical devices included in the ARTG rely on a EU CE Marking approval, it is important that Australian timelines for implementation of regulatory requirements aligning with the EU MDR take into consideration this latest deferment of deadlines.
MTAA continues to work closely with the TGA to ensure that local supply of medical technology is not adversely affected by ongoing COVID-19 challenges and changes in regulatory framework.
Global Medical Technology Alliance Updates
GMTA Proposal for a COVID-19 Thought Partnership Forum
The Global Medical Technology Alliance (GMTA) has unanimously supported a proposal to establish a thought-partnership forum to provide advice, consultation and a coordination mechanism between key global manufacturers of critically needed equipment, the WHO and other multilateral organizations. Read more here.
GMTA Cross-industry Statement in Response to Mexican UN Resolution on COVID-19
On behalf of global biopharmaceutical and medical technologies companies, the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), the International Council of Biotechnology Associations (ICBA), the Global Medical Technology Alliance (GMTA), the Global Diagnostic Imaging, Healthcare IT & Radiation Therapy Trade Association (DITTA), and the Global Self-Care Federation (GSCF) welcome the initiative by the Government of Mexico to highlight the need for effective international cooperation to ensure that no one is left behind in the race against COVID-19 through the UNGA Resolution “International Cooperation to ensure global access to medicines, vaccines and medical equipment to face COVID-19” (A/75/L.56). Read more here.
MTAA temporary Code exemption
As the COVID-19 situation is evolving at a fast pace, MTAA understands that there will be an increased need to support Hospitals and other Healthcare settings (Healthcare Professionals [HCPs]) to ensure they have adequate supplies to be able to care for and treat patients in a healthy and safe environment.
The Code Authority Committee recommended to the Board that a temporary exemption be granted to MTAA members in relation to clause 9.9.4 of the Code of Practice Edition 11 2020 (the Code). This temporary exemption is to assist members to provide medical equipment, technologies and related services to HCPs during the COVID-19 pandemic.
When dealing with any request related to COVID-19, the principles of the Code should always be applied, but in some cases the current situation may require that principles be applied in a different way than in normal times. Therefore, it was proposed to the Board that Members be advised that responses to such requests must:
- Be free from any intent to improperly induce purchase of products or services;
- Be fully documented to allow for full transparency;
- Always consider the perception and the image of the industry;
NOTE: For the purposes of the temporary exemption, the company must document requests related to COVID-19 to the Code Authority.
At the April Board meeting, the Board of Directors resolved to allow the temporary exemption of Members from Clause 9.9.4 of the Code of Practice Edition 11 for the duration of the COVID-19 pandemic.
ACCC exemption update
The ACCC has granted interim authorisation to MTAA to allow its members and other groups, such as suppliers or distributors of medical equipment, to share information between each other, co-ordinate orders and supply requests, prioritise requests, and jointly tender to supply COVID-19 medical equipment.
The ACCC initially granted interim authorisation to MTAA on 25th March. Subsequently MTAA has amended its application, and sought interim authorisation for the conduct described in the amended application. The ACCC has revoked the interim authorisation granted on 25 March, and granted replacement conditional interim authorisation to MTAA for its amended conduct. Read more here.
NSW Government PPE Survey
The NSW Government is seeking to understand the constraints the medical technology industry is experiencing with regards to obtaining personal protective equipment (PPE) or retooling to meet changed or new customer demands.
They have developed a short survey and have requested that it be distributed to MTAA members in order to help the NSW Government target their efforts at addressing the problems for business in accessing what they need to keep their business going such as PPE, sanitiser, parts and raw materials and developing a clear picture of the impact this is having on your business operations and the industry as a whole.
The survey is available through the following survey link.
The story behind your pathology tests
Pathology Awareness Australia Limited (PAA) is a not-for-profit group representing approximately 95% of the Australian pathology landscape. They launched the Know Pathology, Know Healthcare initiative to help educate the Australian public about the critical role that pathology plays in our lives. Know Pathology, Know Healthcare recently launched an animation to show the story behind pathology testing. You can watch it here.
9 April 2020
International air freight options
The Department of Industry, Science, Energy and Resources is working with Austrade and other Australian Government departments to assist Australian importers and exporters in identifying available international air freight opportunities.
Air freight capacity on repatriation international passenger services to and from Auckland, Hong Kong, Los Angeles and London
To support Australian importers and exporters, Qantas and Virgin Australia are offering air freight capacity on repatriation international passenger services to and from Auckland, Hong Kong, Los Angeles and London, from Thursday 9 April 2020.
While these services are limited, this will give Australian producers a way to obtain valuable inputs for their businesses. The return flights to Australia will bring in vital medicines, medical supplies and equipment to support Australia’s response to the COVID-19 pandemic. The confirmed international flights will operate for approximately 4 weeks.
For more information on the repatriation flights, visit:
Qantas or email freightsalessupport@qantas.com.au
Virgin Australia or email syd.cargosales@fly.virgin.com
Additional freight-only flights
In addition, 55 commercial freight-only flights have also been scheduled for April, totalling 540 services for this month.
Austrade advise that the best way to access these flights is to utilise your standard freight operations channels, including your Freight Forwarder.
The Customs Brokers and Forwarders Council of Australia Inc. (CBFCA) represents members interests in international trade logistics and supply chain management service provision. You can refer to this link to help you find a freight forwarder. The International Federation of Freight Forwarders Associations (FIATA) also has a member directory that can be searched through this link.
Austrade
If you are unable to identify air freight opportunities through these channels, Austrade, https://www.austrade.gov.au, have established a dedicated email address for international freight enquiries: Airfreight@austrade.gov.au.
Australian Government’s International Freight Assistance Mechanism
For information on the Australian Government’s International Freight Assistance Mechanism to back Australia’s agriculture and seafood export sectors, visit:
https://haveyoursay.agriculture.gov.au/international-freight-assistance
31 March 2020
Overnight Medtronic announced they are publicly sharing the design specifications for the Puritan Bennett™ 560 (PB 560) to enable participants across industries to evaluate options for rapid ventilator manufacturing to help doctors and patients dealing with COVID-19. Read more.
Local success story: the US FDA has approved Abbott Labs five-minute “rapid coronavirus test” – this machine was developed in Melbourne and for the first few years of its life was exclusively manufactured in Dandenong by SRX. The machine, IDNow, was originally developed for Alere and production was moved from SRX after Alere was purchased by Abbott. Read more.
- MTAA member Gelworks is setting up to start manufacturing hand sanitiser to help out with the local shortage. Read more.
3DMeditech available for local device, part and component collaborations
We are all aware that the ongoing Coronavirus (COVID-19) global health emergency has resulted in acute shortages of key medical devices and component parts. These shortages could be caused by unprecedented demand, logistics constraints or even export controls in the jurisdiction of manufacture.
Early reports from Europe and North America indicate that 3D Printing is uniquely capable of rapid local deployment to assist in preventing these shortages. However, when doing so, ensuring that the highest levels of clinical, engineering, regulatory and supply certainty are met is essential.
Based in Melbourne, 3DMEDiTech (www.3dmeditech.com) operates one of the only – and certainly the largest – ISO13485 certified medical 3DPrinting manufacturing facilities in Australia. Read more.
Fumigation of testing kits
We have been advised that any Customs Broker or Importer that has a consignment that contains COVID-19 diagnostic kits or related goods for Coronavirus that is subject to Brown marmorated stink bug (BMSB) may contact the Seasonal Pest Policy team at SPP@agriculture.gov.au and they will manage the consignment on a case by case basis.
Media Release - Clinical trials are critical in COVID-19 fight
In support of the clinical trials sector during the COVID-19 pandemic, a position statement has been developed by the Research and Development TaskForce (RDTF), sponsored by AusBiotech, Medicines Australia, and the Medical Technology Association of Australia, together with respective memberships. Read more.
Request for Member information - freight
If you are experiencing difficulties with securing freight routes please contact Rachel Fry: rfry@mtaa.org.au. We are in the process of compiling information on this to escalate to government.
25 March 2020
MTAA is working closely with federal and state governments to ensure that the manufacture and supply of life-saving medical technology is maintained throughout the COVID-19 pandemic response.
In addition to providing daily updates to Health Minister Greg Hunt and Industry Minister Karen Andrews, MTAA is a member of the Coronavirus Industry Roundtable which is meeting regularly to discuss and address supply chain issues.
If your company has a specific issue that needs to be raised in the Roundtable process, please contact Rachel Fry at rfry@mtaa.org.au.