MTAA Committees
Committees and their members perform a crucial role in supporting the Board and driving the delivery of MTAA's Strategic Plan and delivery of Association policies in matters pertinent to its experience. These Committees provide a vital link between industry members, the Board and MTAA.
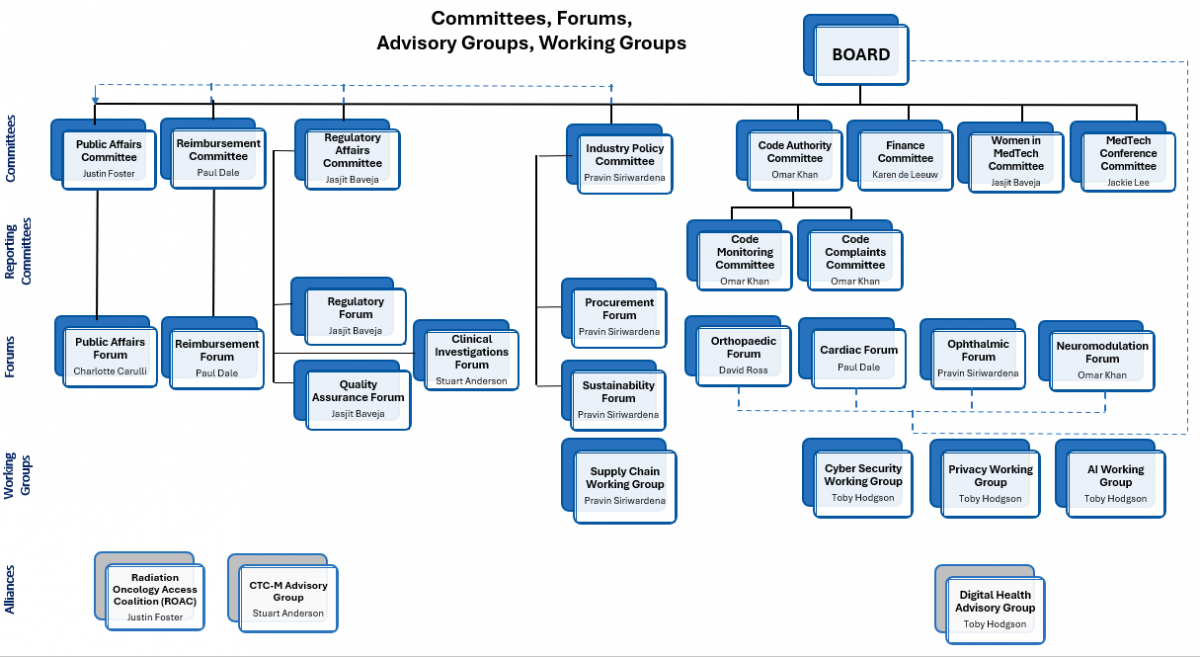
Public Affairs
To provide strategic advice, shared intelligence and coordinated activities to support MTAA’s advocacy efforts on behalf of its members.
The Committee supports MTAA’s direct engagement and media campaign activities, including contributing to patient stories, data and insights on the
effects of positive and negative reforms.
The Committee is also responsible for supporting MTAA with the development and execution of the Advocacy Strategy and its activities, including active stakeholder engagement to promote priorities of the industry and the strategic issues identified by other committees.
The Committee also supports the amplification of MTAA’s advocacy content, which includes reports (such as the Value of MedTech Report, Digital Health Report etc.), submissions and industry content products and messages.
Industry Policy
To promote initiatives (outside regulatory and reimbursement) which impact on industry development and sustainability and healthcare policy.
Reimbursement
To promote the MedTech industry’s access to funding and reimbursement pathways that enable timely public and private market access.
Regulatory Affairs
To develop industry positions and decide on the strategic direction of MTAA on regulatory matters within the scope of the Therapeutic Goods Act, the Medical Devices Regulations and the Advertising Code, alignment with EU Medical Device Regulations and international regulatory harmonisation forums (IMDF).
Code Authority
Responsible for oversight and effective administration of the Medical Technology Industry Code of Practice (Code), including complaints and monitoring.
Code Monitoring
Reviews company monitoring reports to determine compliance with the Code.
Finance
Assist the Board in the effective discharge of its responsibilities for financial reporting, internal controls, audit, compliance and risk management.
Women in MedTech
To drive and fulfil the WIMT mission: “The Women in MedTech (WiMT) mission aims to proactively support gender diversity within the MedTech industry, effectively supporting women to unlock their full potential.”
MedTech Conference
To drive the agenda, sponsorship and attendance of the annual conference that appeals to MedTech industry leaders and executives.